Strategies and Key challenges for Safe Parenteral preparation
ABSTRACT
Parenteral dosage forms differ from all other pharmaceutical dosage forms because they are injected directly into body tissue through the primary protective systems of the human body, the skin and mucous membranes. They have many advantages like routes of administration, elimination of first pass effect, better absorption. They can be in a solution, suspension, emulsions for injection or infusion, powders for injection or infusion, gels for injection and implants. They are sterile preparations that are administrated directly into the systemic circulation of humans or animals. Its unsafe use can transmit various blood borne pathogens. This article aims to discuss the challenges and its importance for safe injection practice.
INTRODUCTION
Certain pharmaceutical agents, particularly peptides, proteins, and many chemotherapeutic agents, can only be given parenterally, because they are inactivated in the gastrointestinal tract when given by mouth. Parenterally administered drugs are relatively unstable and generally highly potent drugs that require strict control of administration to the patient1. Due to the advent of biotechnology, parenteral products have grown in number and usage around the world. Parenteral products are one of the important health care procedures used globally to administer drugs. The establishment of "Safe Injection Global Network (SIGN)" was a milestone towards safe injection practice globally. People perceive injection as a powerful healing tool and do not hesitate to pay more for injections. Rational and safe use of injections can save many lives, but unsafe practice threatens life. Medicine is rigorously tested for safety and effectiveness before becoming available to the consumer. Ensuring safe injection practice is one of the greatest challenges for healthcare system1.
Keywords: strategies, parenteral, injection; interventions, particulate matter, sterility, pyrogen, excipient.
KEY CHALLENGES OF PARENTRAL PREPARATIONS1
- To be clear or practically exempt of visible particle and to be free from sub-visible particles as required by the pharmacopeias’ USP, EP and JP.
- To be sterile.
- To be pyrogen-free.
- Excipients should not adversely affect the intended medicinal action of the drug products, nor at the concentration used to cause toxicity or undue local irritation.
- Intradermal (I.D.) (into subcutaneous tissue)
- Subcutaneous (S.C.) (into skeletal muscle)
- Intravenous (I.V.) (into veins)
- Intra-arterial (I.A.) (into arteries)
- Intrathecal (I.T.) (cerebrospinal fluids)
- Intraperitoneal (I.P.) (Peritoneal cavity)
- Intra-articular (Synovial fluids)
PARTICULATE MATTER
Presence of foreign visible or sub-visible particulate matter in injectable/ parenteral formulations has been one of the most commonly seen reasons for rejections. All injectables are mostly contaminated with some level of particulate matter. This Particulate matter is a critical quality attribute which has direct impact on product safety. Therefore, the United States Pharmacopoeia has defined the standards for particulate matter. These standards are established for all injectable preparations such as large-volume Injections for single-dose infusion and small-volume Injections, solutions for injection administered by intramuscular or subcutaneous route, except parenterals for use as irrigating solutions, radiopharmaceutical preparations and parenteral products for which the labeling specifies use of a final filter prior to administration1.
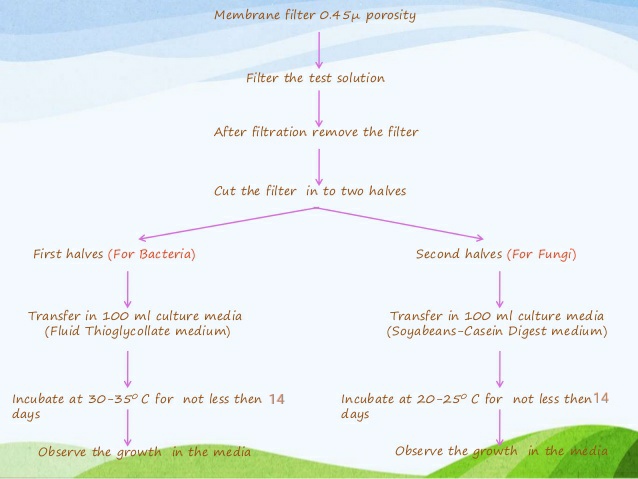
Without sterility testing, no sterile medical preparation may be released onto the market. Parenteral preparations must be sterile means free from the presence of viable microorganism. To identify the viable contamination (bacteria, fungus, spores etc.) in the product sterility testing Is the essential method. As this testing is very important, it also has one limitation that it is time consuming. Once the product sterility was done, it requires 14 days of long incubation time. As we know that bacteria require 3-5 days for the growth and fungus require 5-7 days for growth then why sterility incubation required 14 days. Sterility testing require 14 days of long incubation time because there are some bacteria which are very slow growing like Propionibacterium acne. P. acne is gram positive, rod shaped, slow growing bacteria which is found in the acne of humans. This bacterium is very slow growing, and it could be the source of product contamination. To recover these type of slow growing microorganism, 14 days are enough to support the growth of these microorganism if present in the product. Another reason is that in aseptic environment microorganism could be in damaged or in injured form so it requires long time for the recovery of these microorganism in media1.
Strict requirements apply to sterile formulations, i.e. parenterals intended for injection, infusion or transplantation into the human or animal body. Testing the sterility of the filled final product is an essential and a decisive criterion for approving the release of a complete batch. According to the international pharmacopeias (USP <71>, EP 2.6.1, JP 4:06, WHO (QAS / 11,413 final), sterility testing is not only to be carried out on parenterals, but is also mandatory for the release of eye drops, ointments, creams, aerosols and solids, such as surgical sutures.
The USP describes three general methods for sterility testing:
- Membrane Filtration,
- Direct Transfer (Product Immersion); and
- Product Flush.
Membrane Filtration Sterility Testing
The Membrane Filtration Sterility Test is the method of choice for pharmaceutical products. It is not the method of choice for medical devices; the FDA may question the rationale behind using the membrane filtration test over the direct transfer test for devices.
Direct Transfer Sterility Testing
Combination products: This method is the method of choice for medical devices because the device is in direct contact with test media throughout the incubation period.
Product Flush Sterility Testing
Combination products: The product flush sterility test is reserved for products that have hollow tubes such as transfusion and infusion assemblies where immersion is impractical and where the fluid pathway is labeled as sterile.
PYROGENS
Drugs for injection and medical device products for implantation or other systemic exposure should meet pyrogen limit specifications before they are marketed. Pyrogens are substances that can produce fever when present as contaminants in a drug or medical device. Most pyrogens are biological substances derived from bacteria, fungi, and viruses; material-mediated pyrogens (MMPs), while less common, may also be present. Animal-based pyrogen tests are often conducted to investigate the presence of pyrogens. Rabbit are used to perform the test because their body temperature increases when pyrogen is introduced by the parenteral route1.
Bacterial Endotoxin Test (BET) is useful to determine the harmful pyrogen in pharmaceutical products and water for injection using a gel clot method. Endotoxin are also commonly known as pyrogen and they are mainly produced by gram negative bacteria. The principle of Bacterial Endotoxin Test makes it the most sensitive test that one can use to detect and quantify endotoxins, toxins that are famously known for causing fever in humans. Pharmaceutical products can be contaminated during purification, production and packaging stages and the Bacterial Endotoxin Test is used to confirm that the products are not contaminated before they are administrated for use of humans5,7.
Bacterial Endotoxin Test usually uses three general endotoxin detection method that are usually accepted. There is the gel clot technique that usually measures and detect endotoxins through the gel formation process.
There is the turbidimetric methods that usually detects the amount of endotoxins based on the measuring of turbidity. Development of turbidity indicates that whether a sample contains endotoxins or not.
There is also a chromogenic method that test for development of colour. The development of colour indicative of the presence of endotoxins in a sample.
Bacterial Endotoxin Test is usually easy to perform and one can be able to obtain the results of the test within an hour. It also eliminate the need to use an animal for testing of pharmaceutical products.
EXCIPIENTS
Excipients are an integral part of pharmaceutical products development to achieve desired product proile (stability and eficacy). Parenteral formulations are sterile, pyrogen-free; free of particulate matter and by-pass the body’s natural defense mechanisms. Excipients may demonstrate a synergistic effect when combined with an active ingredient but may also lead to unwanted reactions with the drugs and packaging components. Ideal excipients are required to be considered safe, inert and multifunctional7.
Because of the route of administration for the parenteral dosage form, there are only very limited sets of excipients that have been approved.
To achieve a new excipient approval, you have to ensure there is enough safety data available, and that the toxicity studies have been done. So, if you want to go to anything novel, a whole new set of challenges and additional time and cost need to be covered.
Many formulators therefore tend to use the excipients that already have a precedent in another injectable dosage form. In other words, the choice is limited8.
Too small to be captured by the regular filtering methods used by excipient manufacturers to remove bacteria, endotoxins must instead be eliminated through more complex – and expensive – processes. Consequently, parenteral grade excipients can cost as much as five times more to produce than those for oral formulations9.
CONCLUSION
Parenteral preparations are sterile and pyrogen-free preparations that are designed to be administered directly into the systemic circulation of humans or animals. They should meet the pharmaceutical quality standards described in various pharmacopoeias and ICH guidelines, ensure clinical tolerance and be safe for their intended purpose of use.
ACKNOWLEDGEMENT
The authors are grateful to Mr. Samir Sheth
, M.D, C.E.O, Pharmaceutical Company, Mumbai Mr.
Rajendra Mishra, Technical Director KGN Pharmaceuticals Pvt. Ltd., Mumbai and our
colleagues Mr.
Gaurav Tripathi, Works Manager, Mr. Sanjay Chauhan, Sr. Manager, Supply Chain, and Ms.
Monika Tomar, Manager-R&D, Sigachi
Industries Private Limited, Telangana, India for support.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
QUERY
Ask if you have any Query @ http://pharmasolution1.blogspot.com, I will revert back to you with solution.
REFERENCES
- USP, EP and JP Pharmacopoeia
- United States Pharmacopoeia, chapter 790, Visible Particulates in Injections.
- Tran T, Kupiec T, Trissel L (2006) Quality Control Analytical Methods:Particulate Matter in Injections: What is it and what are the concerns?International Journal of Pharmaceutical Compounding 10: 202-204
- United States Pharmacopoeia chapter 788, Particulate Matter in Injections.
- ICH Q6, Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products
- ICH Q8, Pharmaceutical development
- Pharma Solutions by Dr. Ajay “Impurities in pharmaceutical products How, Why, Characterize and Acceptance criteria”
- Ajay Kumar Singh “Effect of Aqua- Organic medium on ion-dipole type Reactions” in ARJP
- Ajay Kumar Singh “A study of alkaline Hydrolysis of ethyl isonicotinate” in ARJP
- Leon Lachmen Herbert A liebeman,The theory and practice of Industrial Pharmacy.
- Martin’s Physical pharmacy and pharmaceutical sciences.
- Photo courtesy: Research Gate
Abbreviations:
SIGN: Safe Injection Global Network ,USP: United States Pharmacopoeia ,EP :European Pharmacopoeia ,JP: Japanese Pharmacopoeia , I.D.: Intradermal, S.C.: Subcutaneous I.V.: Intravenous ,I.A.: Intra-arterial ,I.T.: Intrathecal ,I.P.: Intraperitoneal , WHO: World Health Organisation, QAS: Quality Assurance Scheme, FDA: Food and Drug Administration, MMPs: Material-Mediated Pyrogens, BET: Bacterial Endotoxin Test, ICH: International Council of Harmonisation, R&D: Research and Development.
LEGAL BARRIER:
A legal barrier to copying of this article.







Comments
Post a Comment