Magnesium stearate: Formulation challenges for oral solid dosage form
ABSTRACT
Excipients, which are an integral part of any formulation, can significantly impact stability, processability, and performance of a dosage form. Some examples are binders, fillers (or diluents), disintegrants, colorants, buffering agents and coatings. One class of functional excipients that are essential in most solid oral dosage forms is “lubricants”. Lubrication plays a key role in successful manufacturing of pharmaceutical solid dosage forms. Although many failures in pharmaceutical manufacturing operations are caused by issues related to selection of lubrication. Selecting a “lubricant” for a formulation requires a systematic approach with careful consideration of the performance of both product and process.
Keywords: excipients, functional excipients performance, hydrophobic, lubricants, selection, hydration state, surface area, particle size
INTRODUCTION
Lubricants are commonly added to help in the tabletting of many formulations. Following compression, a tablet must be ejected out of the tablet press die. Lubricants reduce the friction between the tablet and the die metal surface, which reduces the ejection force and helps to ensure that the tablet is cleanly ejected and without cracking or breakage. Lubricants are added to the powder mixture prior to the tabletting step to ensure that the tablet is ejected properly from the press. The addition of lubricants also affects tablet properties and can affect the behavior of the powder mixture8.
The functionality of a lubricant varies with its manufacturing process, chemical composition, hydration state, blending time, concentration, surface area, causing picking, sticking, capping, decrease in tablet hardness, increase in tablet disintegration times, decrease in rate of dissolution etc8 .Of the tested lubricants, magnesium stearate provided the best increase in flowability even in the low amounts commonly added in formulations. Magnesium stearate is widely used in the production of pharmaceutical tablets, capsules, and powders.
METHOD FOR SELECTION CRITERIA OF LUBRICANTS:
TYPES OF LUBRICANTS:
Hydrophilic:
Generally poor lubricants, no glidant or anti-adherent properties.
Hydrophobic:
Most widely used lubricants in use today are of the hydrophobic category. Hydrophobic lubricants are generally good lubricants and are usually effective at relatively low concentrations. Many also have both anti- adherent and glidant properties. For these reasons, hydrophobic lubricants are used much more frequently than hydrophilic compounds. Of all the lubricants in use, “magnesium stearate” which is hydrophobic in nature, is the most widely used lubricant in the pharmaceutical industry. Magnesium stearate, a metallic salt boundary lubricant, is probably the most commonly used lubricant for pharmaceutical tableting; it is relatively inexpensive, provides high lubrication, has a high melting point, and is chemically stable.1
CHEMICAL COMPOSITION:
Magnesium stearate (Mg-St) is the magnesium salt of stearic acid. Its anhydrous, monohydrate, dihydrate and trihydrate forms have been prepared. Most tableting materials require lubrication to some degree. Only a few drugs and excipients do not require lubrication, but these are exceptions. Magnesium stearate is extremely difficult to study because it is both a complex nature and low concentration in a tablet.
Magnesium stearate is the most commonly used and most effective of all lubricants. Due to its manufacturing process, there are various impurities in magnesium stearate, often causing incompatibilities with APIs. It contains several impurities7 such as magnesium oxide (MgO) and palmitic acid; so, these impurities 7often react with APIs in the solid-state causing stability issues. Magnesium stearate with impurities of magnesium oxide creates an alkaline environment, causing drug degradation, especially for base-sensitive molecules Magnesium Stearate consists of a mixture of several different fatty acids. A composition consisting of Magnesium Stearate to palmitate in a ratio of 25% to 75% respectively is considered to be optimum for “lubricity” and shear properties. Since the availability of commercial product is difficult, company validate their product with the available composition.
EFFECT ON PLASTICALLY DEFORMABLE EXCIPIENTS:
It is generally accepted that magnesium stearate has more negative effects on the hardness of the tablets with more deformable materials than brittle ones2 Plastically deforming materials: cellulose, and starch was clearly affected by the addition of lubricants.
Microcrystalline cellulose is an example of a material with a high lubricant sensitivity, which effect is caused by its plastic deformation behavior during compression. When microcrystalline cellulose, was mixed with magnesium stearate, the tablet strength was weakened significantly as the amount of added lubricant increased3 (0.0-1.0%) as shown in below.4
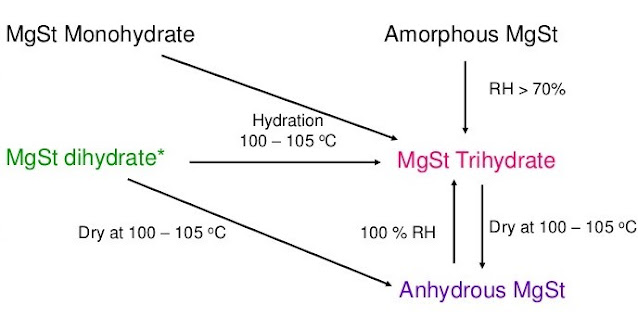
HYDRATION STATE:
EFFECT OF CONCENTRATION AND BLENDING TIME:
Concentration and lubricating time are critical and should be controlled carefully.
If concentrations are too low, or distribution and mixing times are inadequate, problems include i.e.: Picking, Sticking, Capping etc.
If concentrations are too high, or distribution and mixing times are inadequate, problems include i.e., Decrease in tablet hardness, increase in tablet disintegration times, decrease in rate of dissolution etc.
Furthermore, the mixing time for distributing a lubricant is typically up to 5 minutes for better results on compactability and the hardness of tablets. Magnesium stearate is typically added to the blend a few minutes (2-5 minutes) prior to the conclusion of the blending period.
SURFACE AREA AND PARTICLE SIZE:
It is important to understand the impact of surface areas and particle size properties on the performance of the lubricated formulations. The particle size and specific surface area of Magnesium Stearate may be the key factors influencing its lubrication efficiency. The surface area of magnesium stearate is also an important variable and the greater the surface area, the higher the ability to coat other particles in the formulation, ultimately leading to an effective lubrication10. Actually the lubrication efficiency of magnesium stearate improves with increasing its surface area or decreasing its particle size since the increase of surface area can provide more surface coverage. It is also the most likely to cause compression & dissolution problems. Since the lubricating properties of magnesium stearate are related to its ability to coat other particles in a formulation during mixing, samples of a greater surface area should be able to do this more effectively. There is some argument about this however, since it is known that the surface area of magnesium stearate continually changes during blending as a result of its delamination. In practice, the particle size, instead of the surface area, is much more often used as a measure of the lubricant characteristics. It is mainly due to the fact that particle size measurement of a powder material is faster, easier, and less controversial.
RESULTS AND DISCUSSION:
Magnesium stearate has good glidant and anti-adherent properties also. Although it is used in low concentrations; it is often the cause of many problems experienced in solid oral dosage forms. So chemical composition, hydration state, blending time, concentration, surface area and particle size of magnesium stearate must be carefully controlled.
Furthermore, the lubrication efficacy of hydrates of magnesium stearate and their effect on the performance of formulations in pharmaceutical operations were discussed. Overall, it is found that the dihydrate form of magnesium stearate is the best hydration state for lubrication.
In terms of the effect of lubricant particle size, magnesium stearate with a large surface area and small particle size has the best lubrication efficiency, but it reduced the hardness of tablets and caused slow-down of dissolution.
GRAS STATUS:
Magnesium stearate is permitted for use in the European Union and other countries including China, Japan, Australia and New Zealand, and was granted generally recognized as safe (GRAS) status in the United States6.There are no published data available related to the genotoxic potential of magnesium stearate.
CONCLUSION:
Finally, selecting a magnesium stearate for a formulation requires a systematic approach with careful consideration of the performance of both product and process.
This concludes that the selection of magnesium stearate should be based on the following parameters which must be followed in respective finished products.
- Chemical composition of magnesium stearate (Can be validated based on the availability)
- Dihydrated (Due to its crystal structure which is suitable for formulation)
- Concentration and lubricating time (0.25 – 5%, Concentration upto1% gives better results with Max. 5 min. lubricating time)
- Surface area and particle size (Can be validated considering the finished product performance)
- Perrault M, Bertrand F, Chaouki J. An investigation of magnesium stearate mixing in a V-blender through gamma-ray detection. Powder Techn. 2010;200(3):234–45. doi: 10.1016/j.powtec.2010.02.030
- Wang J, Wen H, Desai D. Lubrication in tablet formulations. Eur J Pharm Biopharm. 2010;75(1):1–15. doi: 10.1016/j.ejpb.2010.01.007.
- Jarosz PJ, Parrott EL. Effect of lubricants on tensile strengths of tablets. Drug Dev Ind Pharm. 1984;10(2):259–73. doi: 10.3109/03639048409064649.
- It is based on my personal experiment.
- Sem Image, Inter-Conversion of Mg-St Hydrates – Photo courtesy: Research & Development, Enabling growth of global proportion.
- FDA . 1979. Food and Drug Administration SCOGS Database, Report No. 60; ID Code: 557-04-0.
- Pharma Solutions by Dr. Ajay “Impurities in pharmaceutical products How, Why, Characterize and Acceptance criteria”.
- Pharma Solutions by Dr. Ajay “Tablet processing: Formulation challenges and its solution”.
- Ajay Kumar Singh “Effect of Aqua- Organic medium on ion-dipole type Reactions” in ARJP.
- Ajay Kumar Singh “A study of alkaline Hydrolysis of ethyl isonicotinate” in ARJP.





Comments
Post a Comment